Active Research Projects
“Epigenetics, Polygenic Risk and Social Environment in Autism: A Prospective Study using Smartphone Technology”
Autism spectrum disorder is one of the most perplexing neurodevelopmental conditions facing modern medicine today, with lifelong implication for affected families and society at large. This project will explore the genetic and social environment predictors of autism, using an innovative smartphone app to collect information about infant social development during the first two years after birth. Genetic prediction markers will be compared with social environment factors and how this may affect gene functioning to evaluate the relative contributions to the overall likelihood of autism.
Proposed Role of IRENE: Participating network providers would give information about the study to eligible patients
Principal Investigator: Lane Strathearn, MD
“All of Us Research Program Heartland Consortium”
The University of Iowa is part of a new Midwest consortium that will participate in the National Institutes of Health “All of Us” Research Program. The Heartland Consortium is led by the University of Kansas Medical Center and also includes academic medical centers at the University of Missouri and the University of Nebraska.
The goal of the “All of Us” Research Program is to advance precision medicine research, one day enabling clinicians to tailor patient care by accounting for individual differences in biology, behavior, and environment. The program has created a national research resource that will include comprehensive de-identified health information from more than one million people in the United States.
Unlike research studies that focus on one disease or group of people, “All of Us” is building a broad database that can inform thousands of studies on a variety of health conditions. Researchers will be able to tap into this resource for data to better understand the risk factors for certain diseases and inform future treatments and prevention. “All of Us” follows all federal, state, and local laws in keeping data safe. The program removes all personal details from the data to prevent participants from being identified.
The Heartland Consortium is the first consortium dedicated to engaging participants throughout the Midwest. Together, members of the Heartland Consortium seek to enroll more than 6,000 participants in its first year. In particular, the Heartland Consortium aims to boost the participation of people who live in rural areas and other groups historically underrepresented in research. People who live in these rural Midwestern states experience unique environmental factors that influence health and disease, and that are different from the factors that affect people living and working in urban environments.
Proposed Role of IRENE: Participating network providers would give information about the study to their rural patients
Principal Investigator for UI: Alejandro Comellas, MD
Completed Research Projects
“Rapid Acceleration of Diagnostics COVID-19 Test Us”
From May 2021 through January 2022, IRENE, alongside two other PBRNs (University of Kansas; Kansas Patients and Providers Engaged in Prevention Research, KPPPR and Oregon Health and Science University; Oregon Rural Practice-based Research Network, OPPRN), participated in studies funded by the National Institutes of Health Rapid Acceleration of Diagnostics (RADx) initiative and administered through the University of Massachusetts Chan Medical School (UMass). Participating members took part in two studies:
1) A clinical trial to conduct efficacy testing of a point-of-care (POC) device for SARS-CoV-2 diagnostic tests, and
2) A collection of samples for a biorepository dedicated to COVID-19 research.
This may have been the first time for a group of primary care PBRNs to be involved with a clinical trial for a medical device.
At Iowa, four IRENE members were interested in participating, and staff at each site began preparations for the study. However, as the parameters and requirements of the study continued to shift and change over time, it became much more burdensome to the clinics to continue with their participation. Eventually, just two clinics (Genesis Family Medical Center and West Broadway Clinic) completed the study. Lead clinicians from the four clinics were:
Steve Sorensen, MD and Peter Kim, MD from Genesis Family Medical Center in Davenport, IA
Diana Rabadi-Marar, MD from West Broadway Clinic in Council Bluffs, IA
David W. Carlson, MD from Family Medicine Great River Medical Center in West Burlington, IA
Joshua Baker, DO from West Fork Family Medicine in Rockwell, IA

West Broadway Clinic and the University of Iowa participated in the second part of the RADx initiative, entitled “RADx Tech Test Us Bank.” The goal of this part of the study was to build a bank of samples from individuals who may or may not have the virus that causes COVID-19. The samples are stored to be used by different scientists in the future to study COVID-19 and related viruses. Collection for the study started in June 2021 and ended in January 2022.
Thanks to the hard work of all the clinicians and staff, IRENE enrolled the highest number of participants of all the organizations in the study. Of 1721 patients enrolled, 793 were from IRENE. The next highest enrolling center was UMass at 216 enrolled. On average, participants at IRENE provided 2.21 samples per person, higher than the total study average of 1.76 samples per person. Study participants answered survey questions, got tested for COVID-19 using a mid-turbinate swab, and provided at least one of the following samples for storage and future research: anterior nares swab, mid-turbinate swab, nasopharyngeal swab, saliva sample, dried blood spot, and/or venous blood.


“A Cluster-Randomized Trial Comparing Team-Based vs. Primary Care Clinician-Focused Advance Care Planning in Practice-Based Research Networks”
The study was designed to promote serious illness care planning so that patients with advanced illnesses and limited life expectancies can spend more time at home and receive care concordant with their goals. The Patient-Centered Outcomes Research Institute funded the study, and the lead coordinating site was the Oregon Health and Sciences University. Barcey T. Levy, PhD, MD served as the site principal investigator for the University of Iowa.
Two models for implementing an evidence-based, widely used, and freely available advance care planning program called the Serious Illness Care Program (SICP) were compared in primary care practices across the United States and Canada. The two models were:
1) primary care clinician-focused SICP and
2) team-based SICP.
The core difference was that the primary care clinician alone was responsible for advance care planning in the clinician-focused model. In the team-based model, tasks were purposefully shared across team members with different professions or roles.
Six Iowa Research Network (IRENE) member offices participated in the randomized control trial. Those offices and lead clinicians are listed below.
| ClinicName | Arm |
|---|---|
|
West Broadway Clinic, Council Bluffs, IA |
Team-based |
|
UIHC River Crossing, Riverside |
Team-based |
|
Knoxville Hospital & Clinics, Knoxville |
Team-based |
|
Regional Family Health, Manchester |
Clinician-focused |
|
UIHC Scott Boulevard, Iowa City |
Clinician-focused |
|
UIHC Muscatine, Muscatine |
Clinician-focused |
Patient recruitment for the study ended on September 30, 2020. Advance care planning implementation continues for many family medicine patients and is billable with the CPT code 99497.
IRENE led patient recruitment across the seven practice-based research networks (PBRNs) participating in the study. Those PBRNs included:
Primary Care Research Consortium (PCRC), Durham, North Carolina;
Oregon Rural Practice-based Research Network (ORPRN), Portland, Oregon;
Quebec Practice Based Research Network (QPBRN), Quebec City, Quebec;
State Networks of Ambulatory Practices and Partners (SNOCAP), Aurora, Colorado;
University of Toronto Practice-Based Research Network (UTOPIAN), Toronto, Ontario; and
Wisconsin Research and Education Network (WREN), Madison, Wisconsin.
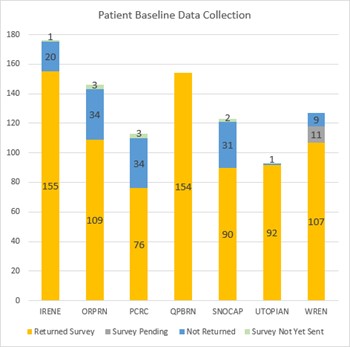
Below is data from baseline recruitment across PBRNs.
Current publications from the study:
1. Totten AM, Fagnan LJ, Dorr D, et al. Protocol for a Cluster Randomized Trial Comparing Team-Based to Clinician-Focused Implementation of Advance Care Planning in Primary Care. J Palliat Med. Sep 2019;22(S1):82-89.
2. Daly JM, Schmidt ME, Thoma KD, Xu Y, Levy BT. How well are serious illness conversations documented and what are patient and physician perceptions of these conversations? J Palliat Care. 2022;Mar 28:1-9.
3. Daly JM, Schmidt ME, Thoma KD, Levy BT. Trained clinician’s documentation of serious illness conversations and use of billing CPT 99497. J Palliat Care. 2021;37(3);323-331.
4. Schmidt ME, Daly JM, Xu Y, Levy BT. Improving Iowa Research Network patient recruitment for an advance care planning study. J Prim Care & Community Health. 2021;12:1-6.
5. Kim P, Levy BT, Daly JM, Berry-Stoelzle M, Schmidt M, Michaels LC, Dorr DA. Prognostic indices for advance care planning in primary care: A scoping review. J Am Board Fam Med. 2020 Mar-Apr;33(2):322-338.